Hydroxychloroquine has been pushed into the limelight far beyond any other drug or treatment because of politics, and for this reason it has become more and more difficult to find the truth about the usefulness of this drug against COVID-19 because most news articles are either sterile and unreadable or so politically charged that you’re not sure if the sky is blue anymore.
As of this week, we’re seeing some results in surveys and clinical studies that are enlightening the field of COVID-19 treatment. Before we dig into the current data, I’d like to discuss why we’re even talking about an anti-malarial being used to treat an RNA virus.
What are Chloroquine (CQ) and Hydroxychloroquine (HCQ)?
Chloroquine was developed by German scientists in the 1930’s as a substitute for quinine to treat malaria, a parasite transmitted through infected mosquito bites that causes flu-like symptoms can creates a high percentage of clinical complications. Because of widespread use when malaria was common it we learned that chloroquine could cause a wide variety of severe side effects, including tinnitus, nausea, vomiting, hallucinations, and even heart failure. In search of a better medication, hydroxychloroquine was developed in the 1950’s which had a lower instances of similar side effects.
Scientists knew the drugs worked since their development but it took until the 2000’s to find the actions of the drugs, and even modern research states that the precise actions of the drugs in living beings are not fully understood. What is known about the drugs it that they have direct effect on a cell’s signaling, ability to clear out old components, and on lysosomes, where cells destroy things from inside the cell. These drugs have broad effects throughout the body, which is likely the reason these drugs carry such a wide variety of side effect. Also because of the wide effects of these drugs they are used not just as antimalarials, but also in the treatment of rheumatoid arthritis, lupus, and a few other diseases. [link]
Of interested to the COVID-19 pandemic is the ability of the drugs to interfere with cell signaling. One of the key aspects of late COVID-19 is a cytokine storm, where the body creates a loop of releasing signals with bad effects that causes further signaling and an explosion of signals in the body that causes over activation of the immune system. Both chloroquine and hydroxychloroquine are capable of slowing down a key signal called interleukin-6 (IL-6) which is a main actor in cytokine storms. Because of this feature of the drugs, doctors began to use them in the early days of the pandemic to treat SARS-CoV-2. [link, link]
Treatments and Early Results
In December of 2019 patients started showing up at hospitals with cases of pneumonia, an unknown cause, and rapid decline in Wuhan, China. The first official case was reported to the World Health Organization (WHO) on December 31, 2019, SARS-CoV-2 was identified as the cause of the disease on January 7, 2020 and the outbreak was declared a Public Health Emergency of International Concern on January 30, 2020. This movement is incredibly fast and would have been unimaginable even 20 years ago, but even after identifying the causal agent of COVID-19 as SARS-CoV-2 medical practitioners were left to treat patients without clear instructions on what drugs to use. [link]
Everyone was grasping at straws to keep patients alive, any drug that might have an effect was being tested in people and studied in cells for potential use. A group of Chinese researchers tested several different treatments on infected cells in culture and published in March of 2020 that remdesivir and chloroquine were able to inhibit the virus in vitro, within cells in culture. [link] As doctors tried anything and everything to save their patients, early use showed that chloroquine could potentially turn some of the worst cases around. People began talking about chloroquine and hydroxychloroquine more and more to the point where even the president of the United States spoke about positive early results with the ominous phrase:
“What do you have to lose?”
President of the United States of America, Donald Trump, April 5, 2020
The Rush for the Drug
Clinical trials were springing up to test any drug that might slow the disease, to the point where medications were getting more difficult to find. Doctors across the world began treating patients with chloroquine and hydroxychloroquine whenever they could, echoing the line of “What do you have to lose?” People were getting sick and dying after they went to church or flew on an airplane. There was non-stop media coverage of the dire consequences of being sick, which led to panic and for some confusing reason a lack of toilet paper. As the world began to follow China’s example to shut down to prevent the spread, scared people were desperate for any good sign and for whatever reason hydroxychloroquine became the go to drug for this disease.
The United States government stockpiled so much of the drug that it caused other governments and companies to rush to purchase some as well. Meanwhile, people with rheumatoid arthritis, lupus, and the other diseases that take these drugs regularly found themselves being cut off. In the background clinical data was beginning to be collected, compiled, and processed to see what effect drugs were having on slowing the spread of COVID-19 and prevent those who were sick from dying.
In mid April, the tide began to turn against the antimalarials, with a clinical trial testing chloroquine in Brazil being stopped due to patient deaths after just 6 days of treatment. Further into the results shows the deaths were primarily in the higher dose category and samples taken from all patients to test for the virus showed minimal efficacy in stopping the virus. [link] Because of the conflict between emotions and data, people who began requesting limited use of the drugs were attacked by those who saw patients recovering faster than expected after treatment. Lives were on the line and the most talked about drug was being shown ineffective in spite of promotion from various officials.
The Slow Process of Science
As time went on studies came out one after another with initial data showing little to no benefit of taking hydroxychloroquine and even some possible risk but the studies were ad hoc collections of data with small patient numbers and limited information that never made headlines. Instead, each passing day the world became more confusing as people couldn’t tell what drugs were helping and which ones were hurting as the world began its first tentative efforts to reopen.
Now we come to this week where a bombshell study was published in the Lancet, a top journal focused on medical sciences. This paper, entitled Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis was a collaborative effort between researchers from prestigious institutions across the United States that compiled information from global cases and meticulously analyzed the data to identify benefits and risks for COVID-19 patients.
For this study, the researchers took all patients that were treated with chloroquine or hydroxychloroquine alone or with a combination of a macrolide, a specific type of antibiotic, within 48 hours of diagnosis. Data was taken from 671 hospitals on six continents from patients hospitalized between December 20, 2019 and April 14, 2020. A total of 98,262 patient records were found within the time frame and were whittled down to 96,032 patients that fit the requirements. According to Johns Hopkins Coronavirus Resource center, a total of 1.976 million people had been diagnosed with the disease by April 14 meaning that the patients this study represents accounted for 5% of all known COVID-19 cases in the world at the time the collection window concluded.
Patients were separated into categories based on treatment and personal characteristics including age, BMI, conditions, and use of drugs to treat COVID-19. This data was standardized, harmonized, and processed to the nth degree to generate the risks associated for a variety of characteristics. All of this data and number crunching is displayed in the two images below, where the risk factors are giving a numerical HR or hazard ratio that shows the increased risk associated with each factor. A hazard ratio is reflective of the risk that each point has, if a condition were to double someone’s risk of death the number would be 2 for mortality HR. Each category receives a dot which represents the calculated hazard ratio with lines around it that show the spread of the possible true number, the narrower the line means the more precise the measurement is.
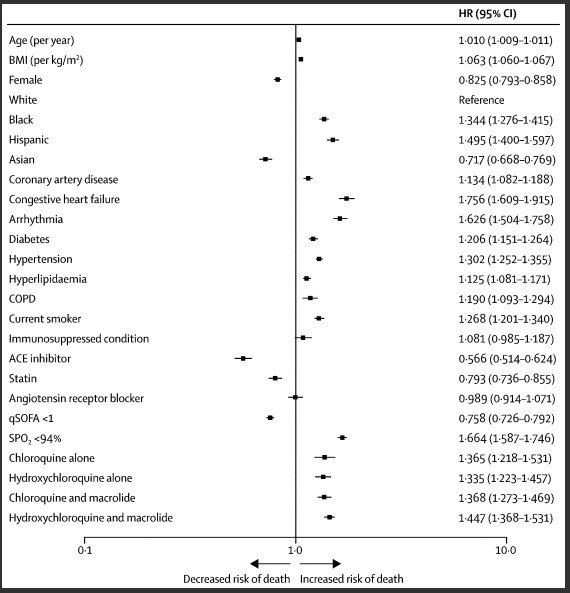
Age and BMI are continuous variables. The 95% CIs have not been adjusted for multiple testing and should not be used to infer definitive effects. ACE=angiotensin-converting enzyme. BMI=body mass index. COPD=chronic obstructive pulmonary disease. HR=hazard ratio. qSOFA=quick sepsis-related organ failure assessment. SPO2=oxygen saturation.
The data isn’t a death knell for the use of antimalarials in COVID-19, but it does show striking evidence. In figure 2 you can observe that the highest risk of mortality comes with having congestive heart failure, furthest shift right, while being on ACE inhibitors appears to be the most protective, furthest shift left. (NOTE: Do not use ACE inhibitors unless instructed to by your physician!) Comparing the medication to other categories shows that someone arriving at a hospital with COVID-19 who also has hypertension is as likely to die as someone who is otherwise totally healthy and is treated with any of the studied drugs or drug combinations. Taking these drugs increases the risk of death close to 35%.

Age and BMI are continuous variables. The 95% CIs have not been adjusted for multiple testing and should not be used to infer definitive effects. ACE=angiotensin-converting enzyme. BMI=body mass index. COPD=chronic obstructive pulmonary disease. HR=hazard ratio. qSOFA=quick sepsis-related organ failure assessment. SPO2=oxygen saturation.
The study continues by looking at the risk of a dangerous known side effect of chloroquine and hydroxychloroquine, the risk of experiencing ventricular arrhythmia, abnormal heartbeats that can cause your heart to pump so fast that it prevents oxygen-rich blood from reaching the brain and can cause cardiac arrest. While the risk of ventricular arrhythmia is spread more between the different treatments, the risk is comparable to having a history of arrhythmia or congestive heart failure. Taking these drugs causes the risk for life threatening arrhythmia to increase by up to 5 fold.
This study was not a blind clinical trial but an amalgamation of patient data that has been carefully sorted through and processed to generate separate risk factors. While this spread of data does not mandate the immediate cessation of all antimalarial treatments for COVID-19, it does show the risks associated with using unproven medicine; especially unproved medicine off label where there is a known risk of side effects.
Hope on the Horizon
Another study published on May 22, 2020 shows data from a clinical trial using remdesivir. [link] This drug originally developed to treat hepatitis C and has been tested against other viral diseases. Remdesivir is able to interfere with viral RNA production, the life blood of RNA viruses such as coronaviruses. The study comes from the National Institute of Allergy and Infectious Diseases and is everything you could want in a study for developing drug efficacy, by including over 1,000 patients, being double blind, randomized, and placebo-controlled the authors are able to remove bias from patients and researchers by comparing the outcome of patients on a drug to the outcome of patients who only think they are taking the drug.
Within this study, the authors show that patients who received remdesivir were able to recover within 11 days while patients on placebo recovered within 15 days. Additionally, treated patients had lower mortality rates and a similar risk of serious adverse events. For comparison, I’ve included a similar figure from the remdesivir paper to highlight the potential benefits of the treatment. While there are some bars that reach into the space where a placebo is better than the treatment, by a majority of measures remdesivir is able to help patients sick with SARS-CoV-2.
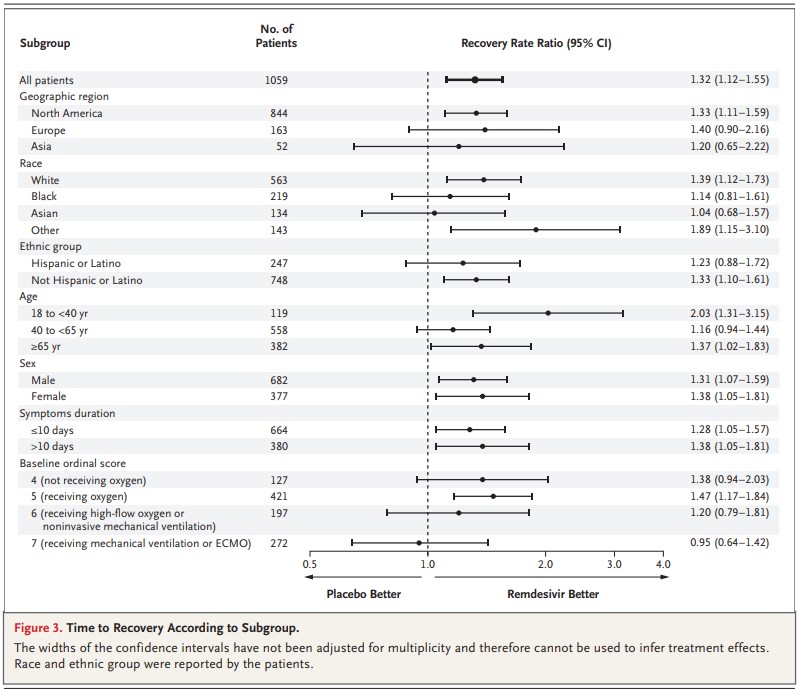
There is no silver bullet, no direct drug or vaccine coming on line any time soon so we will have to keep following the data and following the outcomes as a whole to determine how to save people who are unfortunate enough to get sick with COVID-19. For now, it’s best to social distancing and wear masks to protect yourself and others.
Here’s to a brighter tomorrow,
-Colleen, PMP in the Lab
