With each passing week we grow closer to the conclusion of clinical trials and the eventual release of COVID-19 vaccines. Reporting on the details of what actually occurs in each phase of a clinical trial isn’t consistent, so today’s post will feature an explanation of how a drug goes from an idea to a clinical product.
Research & Development
All new drugs start as an idea. Someone can identify a substance and test it against different conditions or someone can identify a condition they want to treat and try to find the best treatment. Once a drug or condition is identified, then testing begins.
The first hypothesis to tackle is will the drug change the condition in isolation. If your drug is supposed to bind to a molecule and stop it from interacting, you can test interactions in a test tube or other isolated environment. If the drug is supposed to interact with how a cell functions, in vitro studies are performed where cells are cultured in dishes and treated with the drug in question. Both of these tests can be used to figure out if the drug is working properly and there are thousands of papers a year characterizing molecular interactions such as these.
After testing in cells, researchers normally try to replicate the findings in animal studies. By using fish, worms, flies, rats, rabbits, ferrets, pigs, and many other animals researchers can use an animal model to replicate the drugs’ effects. For drugs that target a specific disease, the animal may be genetically or surgically modified to exhibit symptoms. After the animals are prepared scientists perform the treatments and carefully watch the animals to see if the drug works. If any animal becomes too ill they are gently put to sleep to prevent suffering. The goal is to make sure a drug shows the basic traits of being safe and effective before moving on to regulated research.
Research at this stage creates big headlines as scientists find way to cure a very specific cancer in mice. However treating mice in the lab is very different from treating patient populations. When researchers develop animal lines the resulting offspring are usually all as similar as siblings. This means that while a particular animal line may see a very strong effect from a drug, when tested in people that are not related to each other at all the drug is not as effective. Thousands of drugs enter preclinical testing each year but only very few continue on to clinical trials.

Pre-Clinical Research
The purpose of a pre-clinical trial is to establish the safety of a drug. Pre-clinical research is the first stage of research overseen by the FDA in the USA and by other governing bodies around the world. Good laboratory practices, GLP, establish common practices, procedures, and documentation requirements. Worldwide GLP principles include regulation about responsibilities of the staff, the facilities, the materials, and the environment for all experiments. This comprehensive guide to research is necessary to ensure that researchers are performing in good faith and doing good research.
The FDA’s rules governing GLP are in Title 21 Code of Federal Regulation Part 58. [link] These regulations establish the minimum basic requirements for study conduct, personnel, facilities, equipment, protocols, procedures, reporting, and what FDA oversight is done. Animals or cells are tested with different doses of the drug to determine at what level does the drug become toxic. In the case of cellular therapeutics, where a cell is modified and then put back into the body, both the toxicity to the cell and the animal are tested and documented.
Once all of the information is documented and collected, it gets send to the FDA for approval to move to human trials. For every 1,000 candidates that are identified during research, only 2-4% of those drugs ever make it to pre-clinical testing. From the drugs in pre-clinical testing only half are granted permission to begin clinical trials. [link]
Clinical Trials
There are lots, and lots of regulations for human trials [link] and for good reason. It doesn’t matter if the therapeutic in question is natural or synthesized it can still do grievous damage to a body. Just remember that arsenic is as natural as aspirin.
Phase 1 trials are focused on safety in no more than 100 participants. At this stage the trial participants are expected to be relatively healthy adults with few underlying conditions. Here the patients are tested with different doses of the trial drug and observed for any reactions. Doses with strong reactions are removed from further study while other doses are allowed to continue. This change in studied doses actually occured in Moderna’s COVID-19 vaccine trial. [link] Participants are monitored for a few months after exposure to check for signs of lasting harm due to taking the drug.
One large misconception about Phase I clinical trials are that once a therapeutic enters this stage it’s locked into its final form but that is not true. As research continues to evolve, the formulation may adapt to data. For instance if the drug is creating a reaction in all members due to how the active ingredient is packaged, such as a shot or a tablet, then the formulation of the packaging can be changed to minimize the reaction. Clinical trials are a space for ongoing development and fine tuning of the drug in people.
Phase 2 trials are focused on efficacy. This stage of trial recruits 100-300 participants to see if the drug is having the appropriate effect. Different drugs will have different measurable outcomes, vaccines should produce a measurable antibody and T-cell response while cholesterol drugs should lower cholesterol.
Sometimes when the pre-clinical data shows low toxicity and high efficacy phases 1 & 2 are combined and run simultaneously. This helps because the safety is immediately checked in a larger number of people. The only downside is that phase 2 studies take time, typically up to 2 years, but the length can change depending on how long the drug takes to have an effect. Longer trials are also necessary when drugs are expected to have a long duration, such as tracking how long immunity lasts after vaccination.
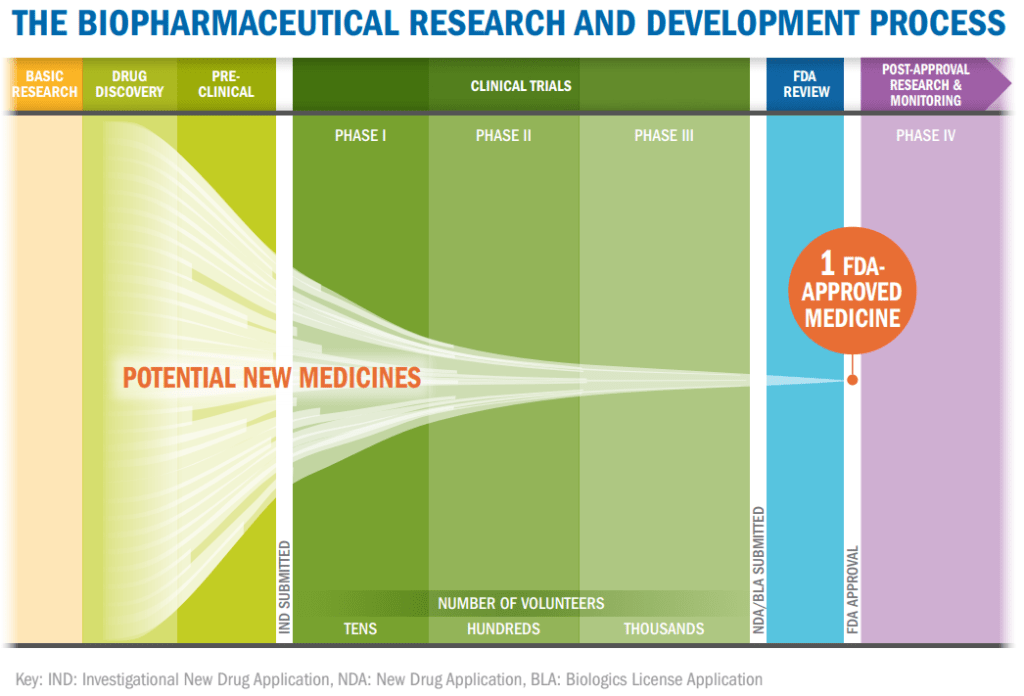
Phase 3 trials use a large number of participants, typically between 300 and 3,000, to monitor efficacy and adverse reactions. Participants in phase 3 trials form the widest pool yet to discover information about side effects such the types, frequency, and severity as well as does the therapeutic work when used in a large, dispersed population. Due to the large amount of data created tracking every person in the study, it usually takes years of monitoring people and tabulating results before a phase 3 study is concluded.
It’s at this stage that placebo controls become most frequent though they can be started in phase 2. A placebo controlled study is one in which there is a patient population who receives the drug that is compared against one who only thinks they are receiving the drug. Placebos come in many forms, from sugar pills to therapeutics created without the active ingredient. Placebo studies can either be single blinded where the participant does not know if they are getting the placebo or they can be double blinded where neither the participant nor the tester know who is getting placebo treatments. By comparing the drug to the packaging, scientists are able to determine if the drug is the source of change or if by taking medication people just think they’re getting better, known as the placebo effect.
Phase 3 trials are the most critical for drugs to move forward as they establish that the drug is both safe and effective in a large patient population. If either point is in doubt, the trial can continue or it may be stopped due to lack of evidence. All drugs completing their phase 3 trials are reviewed by the FDA who will decide to release the drug or if more evidence is needed. Current COVID-19 vaccines in phase 3 testing have 30,000 participants in placebo controlled studies in countries across the world. These studies are so large to try to gather data from many different populations to produce robust results in a limited amount of time.
Phase 4 is an often ignored phase but it is crucial to new drugs on the market. Even after release the drug is still monitored to ensure that safety and efficacy are maintained. This is also a way for companies to support patient populations by offering free doses of a drug during this extended clinical trial phase. Because phase 4 lasts for many years an larger patient population, including regular patients, can be tracked for long term effects. If any adverse consequences are exposed during this time, the drug can be pulled from the market by the FDA.
Why do we go through clinical trials?
Clinical trials are large, multi-year, expensive ventures. Current estimates for the cost of bringing 1 drug to the clinic are $1.2 billion [link] so there’s a major investment to bring any drug out of research to the people that may need it. To offset this, governments give grants to support both research work and clinical trials for drugs that could have widespread use. There’s even a special designation of orphan diseases for diseases that are rare or less profitable to pursue. But with each medication, we want to know they’re properly vetted before they’re handed off for wide spread use.
The last thing we want to do, no matter how well meaning, is begin to knowingly give people medication that causes terrible problems or gives horrific side effects. Through clinical trials we can work to minimize the harm done by trying new therapeutics in a well monitored and regulated way. While harm is never intended, it is a possible outcome of any clinical trial. Even when drugs are used with the best of intentions, side effects can be devastating as seen in the cases of thalidomide and using a Dengue vaccine in children. [link, link]
May patience and perseverance see us through,
–Your friendly neighborhood scientist
