Vaccines are coming into their own this month and the data is starting to flow with information pouring out about efficacy, doses, and who is going to get the vaccines first. After working for 6 years in a group that manufactures mRNA for research use, seeing widespread clinical use of the molecule is a dream come true in what is an otherwise nightmare of a year. Today I hope to share some of my enthusiasm about the work being done with the mRNA based COVID-19 vaccines and assuage some fears that come with any brand new type of therapeutic.
mRNA Therapeutics Development and Manufacturing
Messenger RNA, or mRNA, is a nucleic acid that encodes the instructions for a protein. The DNA in the nucleus is used as a template to make the RNA in a process called transcription that is performed by an enzyme called an RNA polymerase. After the RNA is transcribed from DNA it can be edited to remove parts in a process known as splicing, which generates the finished, translatable mRNA. It’s this finished mRNA that is produced in laboratories for use in research and now manufactured for the clinic.
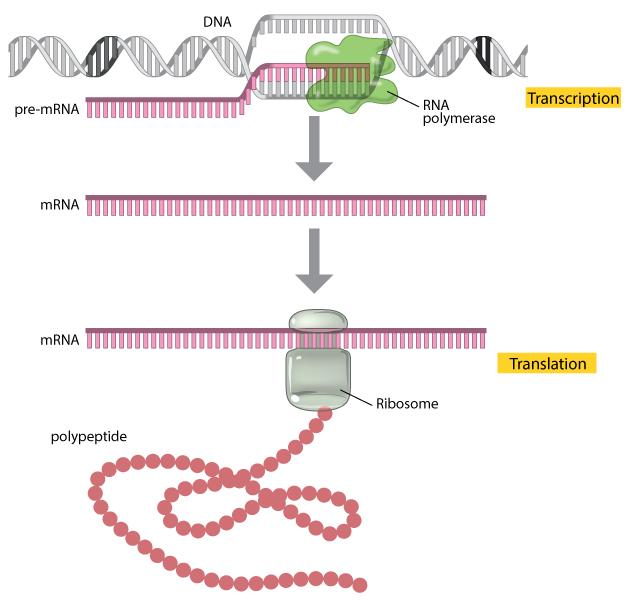
So, how do you manufacture RNA? The steps themselves are pretty simple, the complicated part is the development done to determine the sequence you want as RNA is made of several customizable parts, the cap, the untranslated regions (or UTRs), the coding sequence (or CDS), and the poly A tail. Development of an mRNA product includes adjusting each of these parameters to optimize the RNA’s stability and how well the RNA translates. Once the sequence is locked in, manufacturing begins of large batches.
The manufacturing of therapeutic mRNA is all performed in high end laboratory environments with special air circulations and high standards of environment and documentation, known as Good Manufacturing Practices or GMP. Because mRNA therapeutics are created in a reaction, known as in vitro transcription or IVT, they have a much better purity profile than RNA extracted from cells. Before becoming the final product though, the mRNAs are packaged in lipid nanoparticles (LNPs) that allow for better absorption into cells and delayed release that allows for longer protein expression than naked RNA.

Storing the encapsulated mRNA is tricky and is a major sticking point for the Pfizer vaccine which is currently only allowed to be stored at -80C or -112F. This number comes from when RNA was first being worked with as extracts from cells, and the extreme temperature prevented degradation from residual RNases in the sample that would destroy the product. Encapsulated RNA likely has a different stability profile than naked RNA, and if the encapsulation is disrupted and unable to reform then the drug would be ineffective. There’s every likelihood that Pfizer’s mRNA vaccine is capable of being stored at -20C but unless Pfizer tested their vaccine at that condition it won’t be able to change the storage conditions. These temperature studies, known as stability studies, can last for years and are expensive as you must dedicate finished product to be sacrificed in the study. Moderna has storage conditions based on what they tested, they just happened to start with gentler parameters than Pfizer who decided to take the conservative approach. Don’t worry about the ultra low storage temperatures though, the vaccine will be thawed before it is used.
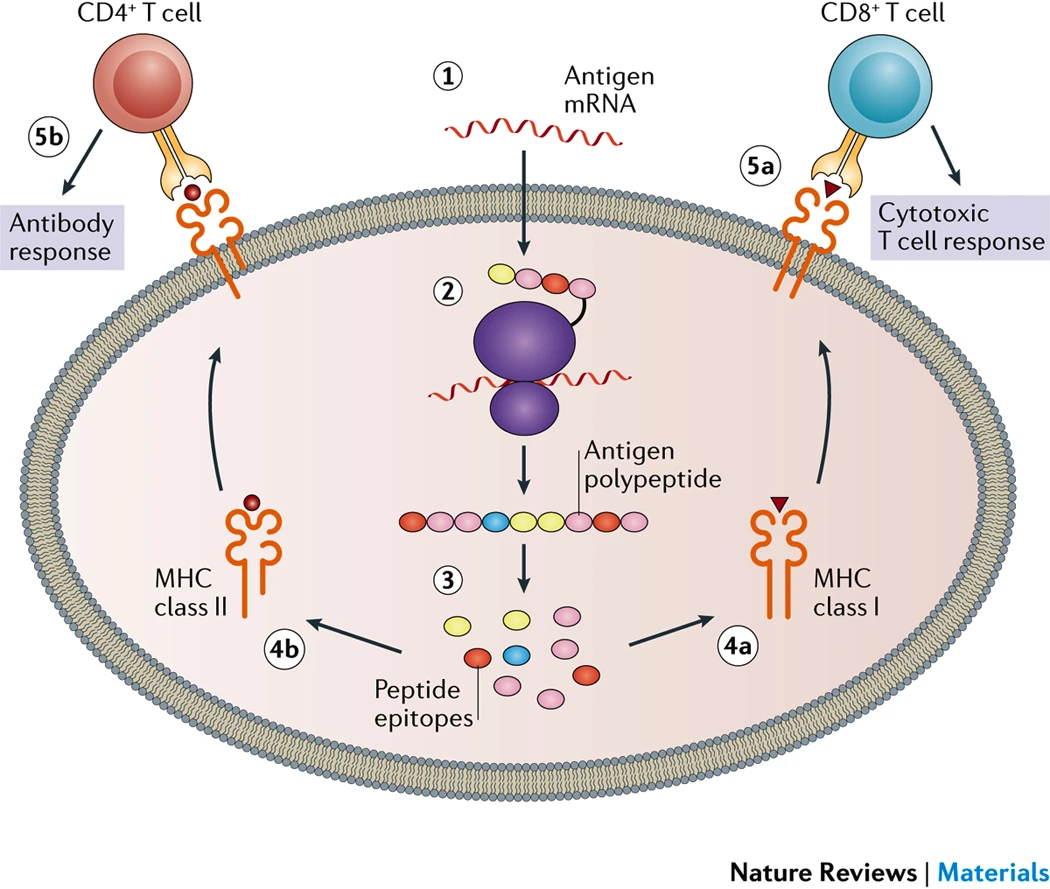
For the current COVID-19 vaccines from Moderna and Pfizer, the spike protein from SARS-CoV-2 is what the mRNAs are designed to express. The spike protein is the critical piece of the SARS-CoV-2 virus that allows the virus to enter cells, so if the body can recognize and attack the spike protein the body should be able to shut down the spike and prevent any viruses from infecting cells. Based on experimental data, minor modifications to the spike sequence were included to stabilize the protein. [link] Once the mRNA enters the cell, it begins to leave the nanoparticle and enter the cytoplasm where the mRNA can be translated into the protein. That protein is then taken and digested by the cell to be examined by the immune system. Once the immune system realizes that the protein on display is not normal, it will begin the immune response where cells develop antibodies against the protein cells memorize the pattern for future attack.
Clinical Trials of mRNA COVID-19 Vaccines
Recently the designs of the COVID-19 vaccine trials have come under attack, but not all of the articles about the trials are set against realistic standards. There was a recent article in Forbes [link] where the author poses unrealistic expectations of how closely participants should be monitored. It’s very easy to talk about what we want to do, but we have to work with what’s practical for a study including 30,000 people. So let’s stay within the set parameters for vaccine trials that have occurred rather than focusing on what we wish could be done.
First and foremost, the number of people in these trials is staggering. For most vaccines there are thousands of people tested, for COVID-19 there are tens of thousands of people in the trials with trials for different vaccines occurring at the same time. The reason to include so many people is to increase your likelihood of finding rare reactions. If 1 out of 500 people experience a reaction, you’ll see it more when you test 1,000 people than if you test 500. Clinical trials leverage what we know about chance occurrences by using statistics to estimate how many people need to be dosed to find rare, uncommon, and common events. This is critical in both the number of people involved in the study as well as how diverse the study population is. Think back to the last time you saw a commercial for medication, all of the side effects that are listed came out of clinical trials like what is currently ongoing.
Population diversity in medical settings is not limited to race, but also includes conditions, age, and general health of the population. Clinical trials start with younger adults who are healthy to prove the relative safety of a treatment before proceeding to more vulnerable populations. With the COVID-19 trials, everyone is watching to make sure as diverse of a set of people are recruited as is feasible to the point where there was a media campaign to help recruit specific groups of people as those who are most affected by the disease were not signing up for the trials. [link] Current exclusions listed on the clinical trials are based on pregnancy, known past SARS-CoV-2 infection, bleeding disorders, immune disorders, and medical treatments so people 18-85 of all races, weights, and vaguely average health status have been included. [Moderna trial, Pfizer trial]
Right now the focus is on adults but will probably include children in future work. Children are different from adults sometimes even down to the cellular level, and those differences in things such as metabolism can change how a drug is processed. We don’t give children regular aspirin for this reason as there’s a higher chance of a bad reaction. There’s every likelihood that a vaccine would need to be adapted to children before widespread use.
In a rare move of general transparency, both Moderna and Pfizer have released their study protocols. This makes reading into the details of the studies much easier as we know essentially the rules each company is playing by during their phase 3 testing. One point that has been made recently is the low number of cases that were needed to determine how effective the vaccine was at preventing disease. Moderna set the interim analysis after 151 cases of COVID-19 were found in the trial and Pfizer set their analysis after 120 cases. These numbers may seem small, especially in light of the 30,000 people enrolled, but they were determined using highly advanced and very precise statistics based on possible efficacy and when they could be certain if a benefit was detected. The true endpoints of these studies are 25 months out from the last participants final dose of vaccine, with results from the studies reported some time after all of the numbers have been crunched.
Interim COVID-19 mRNA Vaccine Results
What we’re working with now are the interim results, from the very earliest we could possibly detect any efficacy signal at all and without the staying power of the final studies with two years of follow up. These interim results made headlines for their high efficacies, but what does that really mean?? It means that they hit a number that should be able to statistically prove there is a difference between the people who receive the vaccine and those who receive placebo, and they were right.
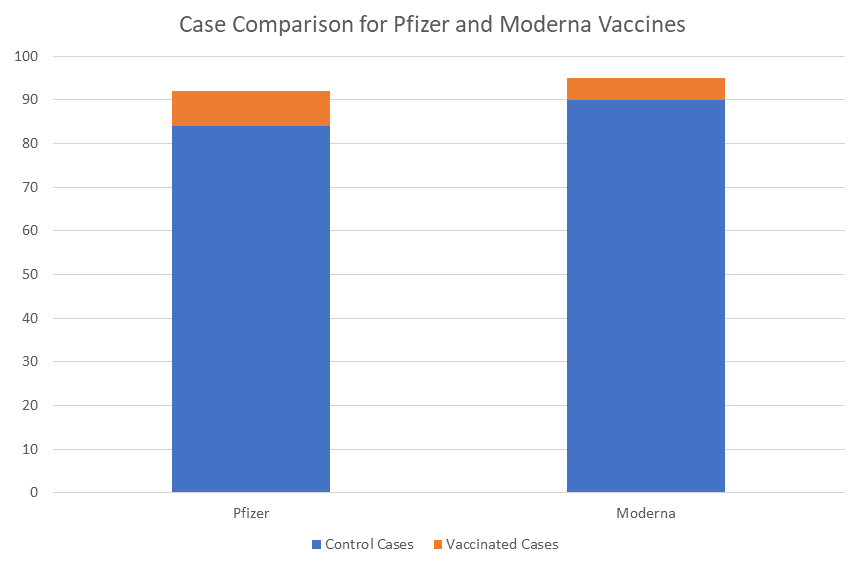
With less than 100 cases in a study, the numbers show that there is an overwhelming propensity for control or placebo recipients to develop COVID-19 vs participants who received vaccine. While there’s a lot of math and statistics to be done, the practical meaning of the work is that if you get a vaccine, you’re 95% less likely to become COVID-19 positive than if you don’t get a vaccine. What’s more, because people who are vaccinated don’t catch SARS-CoV-2 at the same rate as unvaccinated people, we’ll be able to prevent COVID-19 transmission and can establish herd immunity without an enormous portion of the population getting sick. What still needs to be determined is: does this vaccine work for the long term and what are the potential side effects?
Well, based on what we know about lipid nanoparticles and mRNA there’s something that we can say about possible side effects and that’s there just aren’t that many. Most of what has been reported in this and other mRNA vaccine trials are the normal injection site reactions that are mostly mild to moderate. Not everyone will report a difference after the first injection, but nearly everyone reported some kind of symptom after the second injection with symptoms ranging from pain at injection site, to fatigue, chills, and fever. [link, link] Scientists and doctors are now beginning the conversation about how to warn people about the side effects while still promoting the vaccines for their benefits. The greatest fear with any gene therapy is that the new gene may become integrated into the genome, but not to worry because mRNA does not come with that risk. These engineered mRNAs are designed in a way that they are meant to be translated inside the cell and nothing more, there’s no way for the mRNA vaccine to get into the nucleus much less be translated to DNA that can interact with your genome.
mRNA is capable of eliciting a strong immune response, which is what causes the side effects to occur, and shouldn’t cause any long term effects as the lipid nanoparticles and mRNA degrade through normal cell processes within days of entering the cell. Unlike other therapeutics that slowly leave the body, lipids and mRNA are part of a system of natural, fast turnover and the proteins generated from the mRNA have no function outside of the virus they normally come with. With the quick degradation into parts the body is used to seeing, the possible long term effects from the vaccination are minimal. All that being said, participants in the study will be followed for 2 years at minimum to ensure the safety profile is everything we hope it will be.
All of the people in the study are in the same world we are, and while I’m sure there are some people who enrolled in the trial and stopped all precautions, the people I know in the trials are continuing as though they had never been vaccinated at all. Officially, according to phase 1 trial data, it takes until 2 weeks after the second vaccine dose before antibody titers match those of people who have recovered from COVID-19. So anyone who receives a vaccine should continue taking precautions until 2 weeks after their second dose and should continue wearing a mask and distancing until there’s minimal spread in their own community.
When to get vaccinated
The decision to get vaccinated at all for COVID-19 is one currently up for debate, but that debate needs to be tempered with the availability of doses. Because mRNA is such a new technology to be used as a therapeutic there are no commercial scale manufacturing facilities ready to go, everything is being built and brought online Now. We’re not going to have vaccines for everyone until mid 2021 at earliest [link] and someone who can get a vaccine at that point in time may not want to get one. While different states and different countries may have slightly different priorities, everyone is going to vaccinate their healthcare workers first. These are the people who face COVID-19 exposure on a daily basis and are the people we need to be healthy and working to treat those who are sick. Also in the high priority category are people at high risk and those who live in shared facilities where we have seen SARS-CoV-2 spread quickly with deadly repurcussions.
After phase 1 there’s more room for negotiation. The World Health Organization recommends a few plans based on the prevalence of spread while The National Academies has no such distinction. [link, link] What it comes down to is who is next in line when the next doses become available and what do we know about the safety profile at that point in time? That all depends on your personal risk tolerance and your need. For someone who is able to work from home and do most of their shopping remotely, there’s less need than for a first responder who never knows what the situation will be on their next call. As time progresses and vaccinations become more available, we’ll know more about the safety profile of the mRNA vaccines and have some additional options.
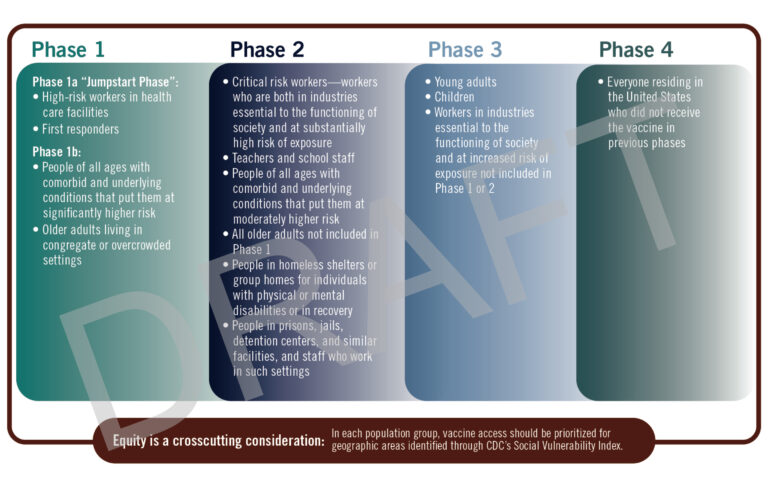
For me, a researcher in the field, I’m going to get the vaccine after I know all of the doctors, nurses, and patient facing people in my hospital system have been vaccinated as I don’t have direct contact with patients and am able to take precautions against COVID-19 fairly easily. Someone who lives with the elderly, has conditions that make them especially vulnerable to COVID-19 may want to get the vaccine earlier than those without. Another large focus should be on teachers and to allow them priority to get vaccinated because we know that kids are able to spread the disease quickly when they don’t feel sick themselves. If you’re not sure about whether or not you should get the vaccine, talk with your doctor to make the right choice for you.
I know we all want to get back to normal as soon as possible, but vaccines aren’t going to magically sweep away this disease. We’re going to have to use vaccination as a tool combined with masking, distancing, hygiene, and the continued sacrificing of what we want to do before COVID-19 will be nothing more than a bad memory and 2020 a dark year in the past.
The end is coming, it’s just not here yet.
Stay strong and stay masked,
–Your friendly neighborhood scientist

2 thoughts on “COVID-19 Vaccines, what’s in an mRNA?”