Headlines over the weekend bickered back and forth over the FDA’s decision to only approve COVID-19 vaccine booster shots to the over 65 and immunocompromised populations. Many promises had been made about possible future booster shot availability but only now are we starting to see the data that tells us just how effective booster shots can be and for whom they can be most effective.
Argument For Boosting
The most compelling argument for booster shots to date comes out of Israel, where there is now widespread adoption of a 3rd vaccine shot at least 6 months after receiving the 2nd shot on the normal schedule of 3 weeks for the Pfizer-BioNTech vaccine. After reviewing the slides shown at the September 17th, 2021 FDA Advisory committee meeting there’s very compelling evidence for ages 60+ that can be summarized in two of their figures. [link]
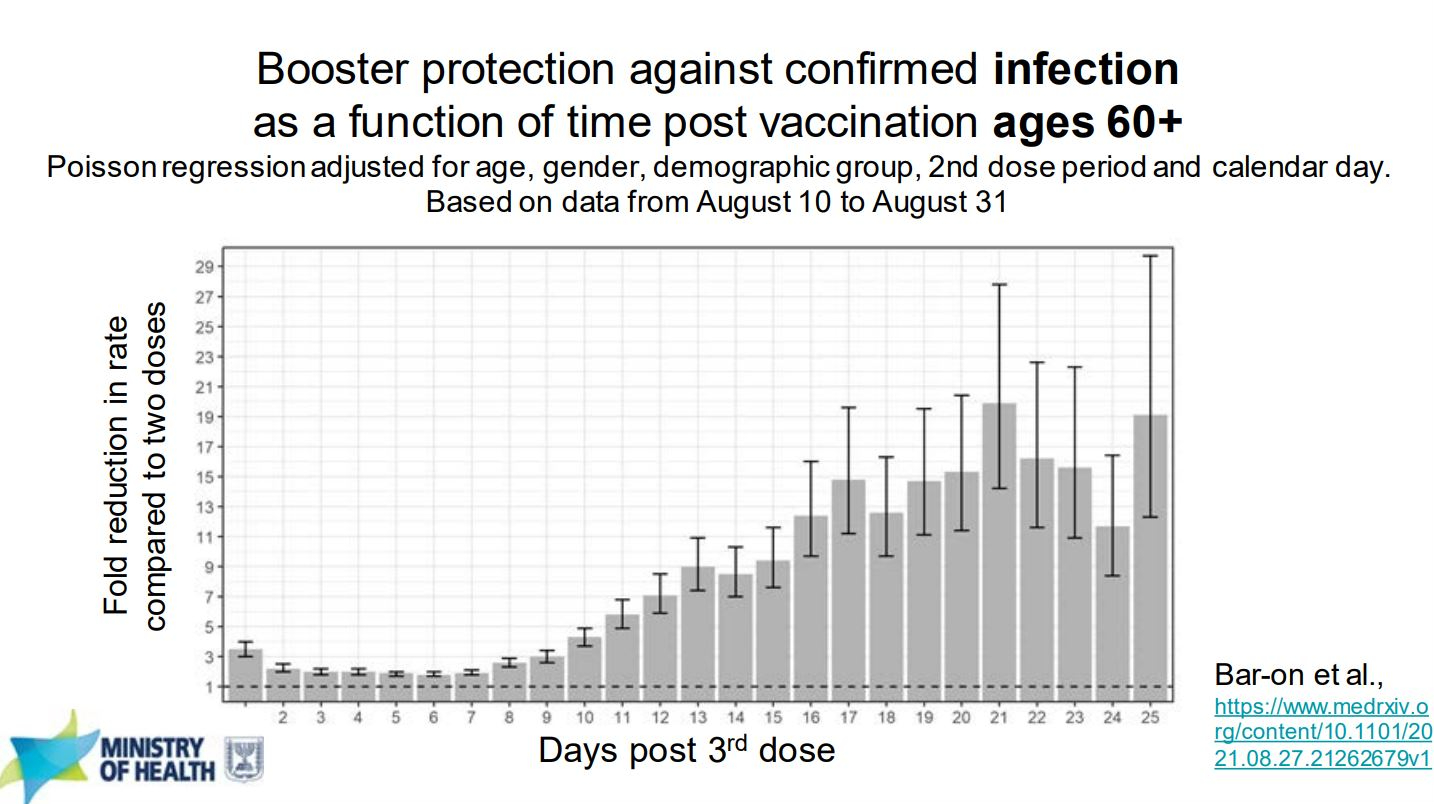
The first figure I want to point out compares the rate of cases in people 60 years of age or older who received a 3rd vaccine dose to those who did not. The effect begins to take place roughly 10 days after receiving the 3rd shot and the effect continues through the monitored day 25 with reduction means reaching a 19 fold difference. This means that for every one person with the 3rd dose who tests positive you would on average see 19 patients with just 2 doses. Such a clearly significant change that running the statistics isn’t necessary.
What is also important to note are the error bars across the graph. These show the range that the real result could fall in, which becomes fairly wide after day 16 and shows the real reduction in rate could be anywhere between 11 and 29 by day 25. While the data still remains positive, because of the wide range we can’t be sure of exactly how effective a 3rd boost is. One of the reasons that could be is that even with a 3rd dose there could be people who still don’t develop an immune response while others could see a huge benefit.
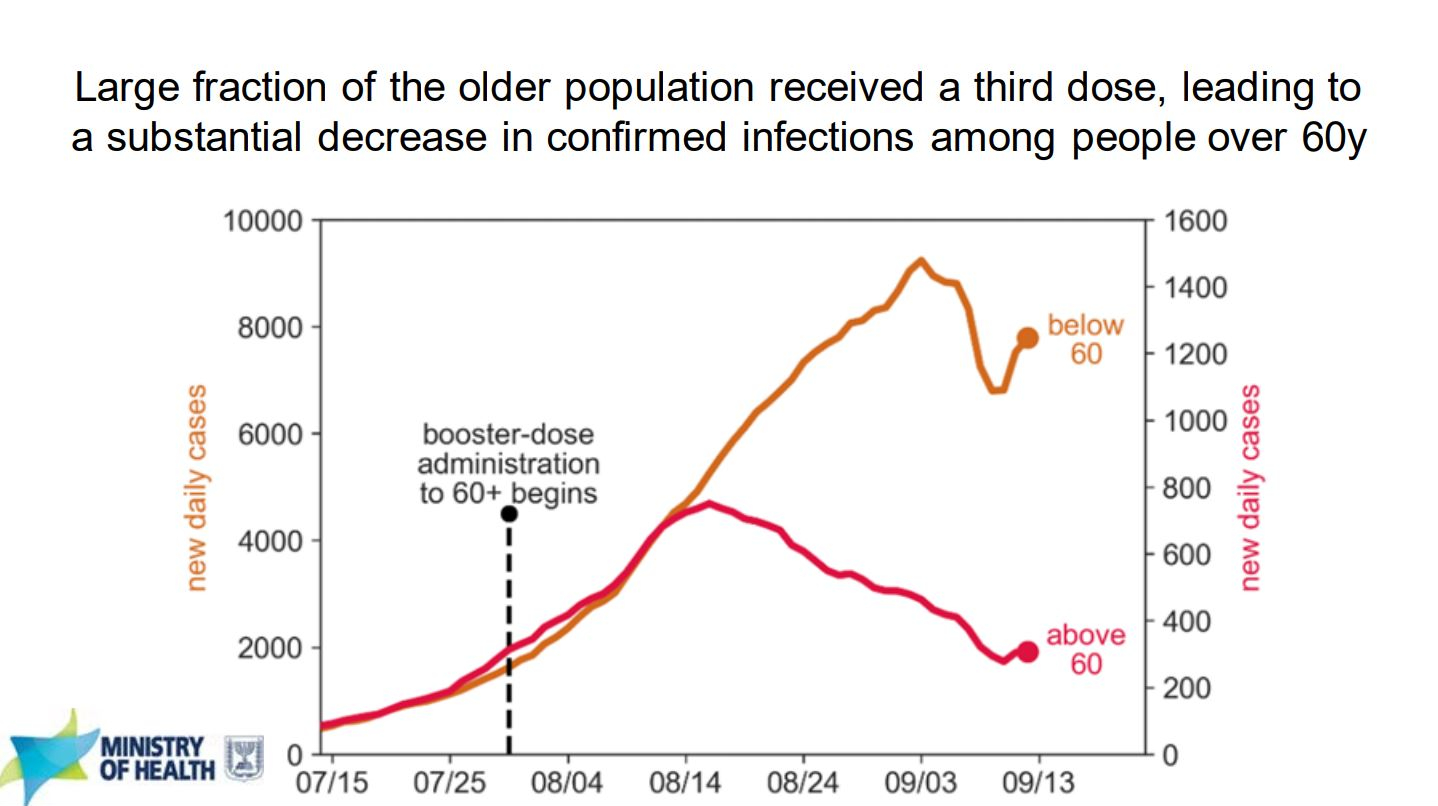
This second figure shows how many cases occurred for the above and below age 60 populations in Israel before and after booster shots were given to the 60+ population. While the figure does show a clear decrease in the number of cases for the 60+ population able to receive the booster shots, it is important to note that the scales for the two populations are very different. The change in scales makes sense because the number of people below 60 years old greatly outnumber those above 60 but it would have been nice to see both numbers normalized to their respective population size rather than presented on different scales. That’s why you see a lot of new daily case numbers normalized to “per 100,000 people”, it makes comparisons between different size populations a lot easier without invoking statistics.
Both of these figures, just two slides out of many, paint a clear image that for the 60+ population of Israel, receiving a 3rd shot of Pfizer-BioNTech’s COVID-19 vaccine had clear clinical benefits. Missing from these analysis is the effect the third shot can have on younger populations because Israel has been limiting access to the shot to prioritize older people first. Give it another few months and there should be data available on how booster shots affected the Israeli population as a whole.
Argument Against Boosting
The data in the previous section is why we’ve heard so much about booster shots, but they present a picture centered around an older population. Data presented at Friday’s meeting augments the Israeli data and shows why the FDA panel recommended limited boosters rather than the widespread ones Pfizer-BioNTech was likely hoping for. [link]
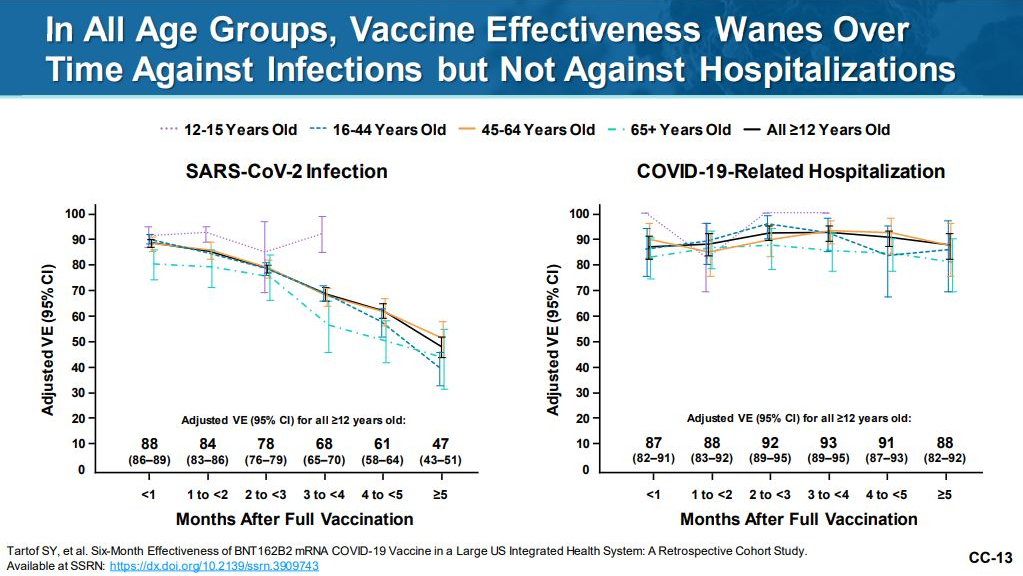
Pfizer-BioNTech had the opportunity to present at the FDA meeting on September 17th and showed great background information with excellent data. The above figure I found particularly striking in that the current 2 dose regiment for their COVID-19 vaccine maintains protection against COVID-19 related hospitalization even while protection against COVID-19 infections declined for all age groups 16 and older. Curiously we get a peek at the long term data for the 12-15 age range which shows fairly steady maintenance of protection against SARS-CoV-2 infection. This is a good place to work in a reminder that even cases of COVID-19 that don’t wind up in the hospital can cause what a layperson would consider fairly severe symptoms of barely able to leave bed or breathe comfortably while also still having a risk of causing long COVID. Even what is clinically termed as mild to moderate COVID can still be a very difficult experience for the person suffering through it.

Another point of evidence brought by Pfizer-BioNTech was how much a 3rd dose of their COVID-19 vaccine could increase the neutralizing antibody titer compared to the amount measured after the second dose. With this data it can be seen that while the third dose does indeed show an increase in the amount of neutralizing antibodies present, there is clearly less of an impact for the 18-55 year old group than there is for the 65-85 group. There is even one patient in the older cohort who went from being at the lower limit of detection (LLOD) after their 2nd dose to clustering in with all the other members after their 3rd dose. Additionally, it is really beneficial to see that even in these limited samples, 11 or 12 people in each age group, that the vaccine is roughly as protective against the SARS-CoV-2 Delta variant as it is against the original strain or which it was designed.
One thing to keep in mind is that we have yet to define what a protective level of neutralizing antibodies is for COVID-19, so we’re not sure what target we should be going for. It may be that the levels displayed after the 2nd dose are enough to protect from a majority of infections and nearly all hospitalizations but we just don’t have the data to prove it yet. Finally, there was no data from Pfizer-BioNTech that showed clinical significance for anyone younger than 65 and relied on the Israeli data for the clinical significance of the 65 and up population.
FDA’s Conclusion (As of September 17, 2021)
The main thing the FDA does is make decisions on the data available and not what the data might be in the future. Politicians, sales representatives, and even scientists can get out there and promote what they see as the possible future for a given product or vaccine but the FDA is tied to the data it is given. Based on strong data from Israel that showed clear clinical significance of a 3rd shot preventing cases of COVID-19 they approved the vaccine for that population. Because there was no clinically significant data shown for a large population outside of that age range, the FDA decided to limit who would be eligible for the booster shot at this time. That’s not to say that it won’t be available for younger people in the future, or even those who may be at high risk if they catch COVID-19 such as medical and other emergency personnel just as the FDA currently cleared the booster for those who are immune compromised and follow the same trends as 65+ populations.
Based on the full presentations and data I read, I know that if pregnant women such as myself are placed on the eligible list for booster shots I’ll try to get one at my next doctor’s appointment. (Safety information not discussed on this post showed that the risks for the 3rd shot were extremely similar to the risk for the 2nd shot). For those of us who are worried about catching COVID-19 even after being fully vaccinated, there’s no reason right now to rush out and get a 3rd shot because we don’t know if it really helps. Currently the best thing to do is encourage anyone you know who has yet to vaccinate to get their shot and to wear a mask when the situation calls for it.
Here’s hoping for better news soon,
-Your Friendly Neighborhood Scientist
